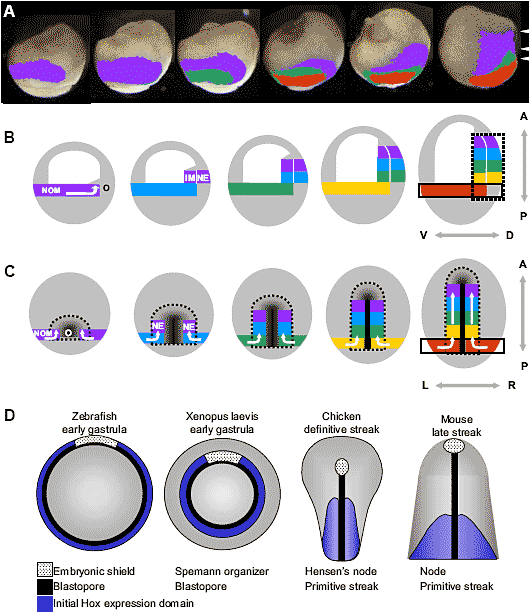
|
AGATHON, A., THISSE, C. and THISSE, B. (2003). The molecular nature of the zebrafish tail organizer. Nature 424: 448-452.
AKAM, M. (1987). The molecular basis for metameric pattern in the Drosophila embryo. Development 101: 1-22.
AVANTAGGIATO, V., ACAMPORA, D., TUORTO, F. AND SIMEONE, A. (1996).
Retinoic acid induces stage-specific repatterning of the rostral central nervous system. Dev Biol 175: 347-357.
BEDDINGTON, R. S. (1994). Induction of a second neural axis by the mouse node. Development 120: 613-620.
BEDDINGTON, R. S. AND ROBERTSON, E. J. (1998). Anterior patterning in
mouse. Trends Genet 14: 277-284.
BEL-VIALAR, S., ITASAKI, N. AND KRUMLAUF, R. (2002). Initiating Hox gene expression: in the early chick neural tube differential sensitivity to FGF and RA signaling subdivides the HoxB genes in two distinct groups. Development 129: 5103-5115.
BELAND, M. AND LOHNES, D. (2005). Chicken ovalbumin upstream promotertranscription factor members repress retinoic acid-induced Cdx1 expression. J Biol Chem 280: 13858-13862.
BERTOCCHINI, F., SKROMNE, I., WOLPERT, L. AND STERN, C. D. (2004).
Determination of embryonic polarity in a regulative system: evidence for
endogenous inhibitors acting sequentially during primitive streak formation in the chick embryo. Development 131: 3381-3390.
BERTOCCHINI, F. AND STERN, C. D. (2002). The hypoblast of the chick embryo positions the primitive streak by antagonizing nodal signaling. Dev Cell 3: 735-744.
BLUMBERG, B., BOLADO, J., JR., MORENO, T. A., KINTNER, C., EVANS, R. M.
AND PAPALOPULU, N. (1997). An essential role for retinoid signaling in
anteroposterior neural patterning. Development 124: 373-379.
BORTIER, H. AND VAKAET, L. C. (1992). Fate mapping the neural plate and the intraembryonic mesoblast in the upper layer of the chicken blastoderm with xenografting and time-lapse videography. Development Suppl: 93-97.
BROWN, J. M. AND STOREY, K. G. (2000). A region of the vertebrate neural plate in which neighbouring cells can adopt neural or epidermal fates. Curr Biol 10: 869-872.
BUNDY, J., ROGERS, R., HOFFMAN, S. AND CONWAY, S. J. (1998). Segmental
expression of aggrecan in the non-segmented perinotochordal sheath underlies normal segmentation of the vertebral column. Mech Dev 79: 213-217.
CALLEBAUT, M., HARRISSON, F. AND BORTIER, H. (2001). Effect of gravity on the interaction between the avian germ and neighbouring ooplasm in inverted egg yolk balls. Eur J Morphol 39: 27-38.
CALLEBAUT, M., VAN NUETEN, E., HARRISSON, F. AND BORTIER, H. (2004).
Induction and improved embryonic development by the nucleus of Pander in
associated avian blastoderm parts: influence of delta or gamma ooplasm. J
Morphol 260: 201-208.
CAMBRAY, N. AND WILSON, V. (2002). Axial progenitors with extensive potency are localised to the mouse chordoneural hinge. Development 129: 4855-4866.
CATALA, M., TEILLET, M. A., DE ROBERTIS, E. M. AND LE DOUARIN, M. L.
(1996). A spinal cord fate map in the avian embryo: while regressing, Hensen’s node lays down the notochord and floor plate thus joining the spinal cord lateral walls. Development 122: 2599-2610.
CHARITE, J., DE GRAAFF, W., CONSTEN, D., REIJNEN, M. J., KORVING, J. AND DESCHAMPS, J. (1998). Transducing positional information to the Hox genes: critical interaction of cdx gene products with position-sensitive regulatory elements. Development 125: 4349-4358.
CHAWENGSAKSOPHAK, K., DE GRAAFF, W., ROSSANT, J., DESCHAMPS, J.
and BECK, F. (2004). Cdx2 is essential for axial elongation in mouse development. Proc Natl Acad Sci USA 101: 7641-7645.
CHIPMAN, A. D., ARTHUR, W. AND AKAM, M. (2004). A double segment
periodicity underlies segment generation in centipede development. Curr Biol 14: 1250-1255.
COPF, T., SCHRODER, R. AND AVEROF, M. (2004). Ancestral role of caudal genes in axis elongation and segmentation. Proc Natl Acad Sci USA 101:
17711-17715.
CORDES, R., SCHUSTER-GOSSLER, K., SERTH, K. AND GOSSLER, A. (2004).
Specification of vertebral identity is coupled to Notch signalling and the segmentation clock. Development 131: 1221-1233.
COWAN, C. R. AND HYMAN, A. A. (2004). Centrosomes direct cell polarity
independently of microtubule assembly in C. elegans embryos. Nature 431: 92-96.
COX, W. G. AND HEMMATI-BRIVANLOU, A. (1995). Caudalization of neural fate by tissue recombination and bFGF. Development 121: 4349-4358.
CRAWFORD, M. (2003). Hox genes as synchronized temporal regulators: implications for morphological innovation. J Exp Zoolog B Mol Dev Evol 295: 1-11.
DAVIS, R. L., TURNER, D. L., EVANS, L. M. AND KIRSCHNER, M. W. (2001).
Molecular targets of vertebrate segmentation: two mechanisms control segmental expression of Xenopus hairy2 during somite formation. Dev Cell 1: 553-565.
DESCHAMPS, J. AND WIJGERDE, M. (1993). Two phases in the establishment of HOX expression domains. Dev Biol 156: 473-480.
DIEZ DEL CORRAL, R., BREITKREUZ, D. N. AND STOREY, K. G. (2002). Onset
of neuronal differentiation is regulated by paraxial mesoderm and requires
attenuation of FGF signalling. Development 129: 1681-1691.
DIEZ DEL CORRAL, R., OLIVERA-MARTINEZ, I., GORIELY, A., GALE, E.,
MADEN, M. AND STOREY, K. (2003). Opposing FGF and retinoid pathways
control ventral neural pattern, neuronal differentiation and segmentation during body axis extension. Neuron 40: 65-79.
DUBOULE, D. (1994). Temporal colinearity and the phylotypic progression: a basis for the stability of a vertebrate Bauplan and the evolution of morphologies through heterochrony. Development Suppl: 135-142.
DUBOULE, D. (1995). Vertebrate Hox genes and proliferation: an alternative pathway to homeosis? Curr Opin Genet Dev 5: 525-528.
DUBRULLE, J., MCGREW, M. J. AND POURQUIE, O. (2001). FGF signaling
controls somite boundary position and regulates segmentation clock control of spatiotemporal Hox gene activation. Cell 106: 219-232.
DUBRULLE, J. AND POURQUIE, O. (2004). fgf8 mRNA decay establishes a
gradient that couples axial elongation to patterning in the vertebrate embryo. Nature 427: 419-422.
DURSTON, A. J., TIMMERMANS, J. P., HAGE, W. J., HENDRIKS, H. F., DE
VRIES, N. J., HEIDEVELD, M. AND NIEUWKOOP, P. D. (1989). Retinoic acid
causes an anteroposterior transformation in the developing central nervous
system. Nature 340: 140-144.
ENSINI, M., TSUCHIDA, T. N., BELTING, H. G. AND JESSELL, T. M. (1998). The control of rostrocaudal pattern in the developing spinal cord: specification of motor neuron subtype identity is initiated by signals from paraxial mesoderm. Development 125: 969-982.
FERNANDEZ-GARRE, P., RODRIGUEZ-GALLARDO, L., GALLEGO-DIAZ, V.,
ALVAREZ, I. S. AND PUELLES, L. (2002). Fate map of the chicken neural plate
at stage 4. Development 129: 2807-2822.
FIGDOR, M. C. AND STERN, C. D. (1993). Segmental organization of embryonic diencephalon. Nature 363: 630-634.
FOLEY, A. C., SKROMNE, I. AND STERN, C. D. (2000). Reconciling different models of forebrain induction and patterning: a dual role for the hypoblast. Development 127: 3839-3854.
FOLEY, A. C., STOREY, K. G. AND STERN, C. D. (1997). The prechordal region lacks neural inducing ability, but can confer anterior character to more posterior neuroepithelium. Development 124: 2983-2996.
FORLANI, S., LAWSON, K. A. AND DESCHAMPS, J. (2003). Acquisition of Hox
codes during gastrulation and axial elongation in the mouse embryo. Development 130: 3807-3819.
FRASER, S. E. AND STERN, C. D. (2004). Early rostrocaudal patterning of the mesoderm and neural plate, In Gastrulation: from cells to embryo, C. D. Stern, ed. (New York: Cold Spring Harbor Press), pp. 389-401.
FREITAS, C., RODRIGUES, S., CHARRIER, J. B., TEILLET, M. A. AND PALMEIRIM, I. (2001). Evidence for medial/lateral specification and positional information within the presomitic mesoderm. Development 128: 5139-5147.
GARDNER, R. L. AND DAVIES, T. J. (2003). The basis and significance of prepatterning in mammals. Philos Trans R Soc Lond B Biol Sci 358: 1331-1339.
GAUNT, S. J., DEAN, W., SANG, H. AND BURTON, R. D. (1999). Evidence that Hoxa expression domains are evolutionarily transposed in spinal ganglia and are established by forward spreading in paraxial mesoderm. Mech Dev 82: 109-118.
GAUNT, S. J. AND STRACHAN, L. (1994). Forward spreading in the establishment of a vertebrate Hox expression boundary: the expression domain separates into anterior and posterior zones and the spread occurs across implanted glass barriers. Dev Dyn 199: 229-240.
GERARD, M., ZAKANY, J. AND DUBOULE, D. (1997). Interspecies exchange of a Hoxd enhancer in vivo induces premature transcription and anterior shift of the sacrum. Dev Biol 190: 32-40.
GERHART, J. (2004). Symmetry breaking in the egg of Xenopus laevis, In
Gastrulation: from cells to embryo, C. D. Stern, ed. (New York: Cold Spring
Harbor Press), pp. 341-351.
GLINKA, A., WU, W., ONICHTCHOUK, D., BLUMENSTOCK, C. AND NIEHRS, C.
(1997). Head induction by simultaneous repression of Bmp and Wnt signalling
in Xenopus. Nature 389: 517-519.
GONT, L. K., STEINBEISSER, H., BLUMBERG, B. AND DE ROBERTIS, E. M.
(1993). Tail formation as a continuation of gastrulation: the multiple cell
populations of the Xenopus tailbud derive from the late blastopore lip. Development 119: 991-1004.
GOULD, A., ITASAKI, N. AND KRUMLAUF, R. (1998). Initiation of rhombomeric Hoxb4 expression requires induction by somites and a retinoid pathway. Neuron 21: 39-51.
GRANDEL, H., LUN, K., RAUCH, G. J., RHINN, M., PIOTROWSKI, T., HOUART,
C., SORDINO, P., KUCHLER, A. M., SCHULTE-MERKER, S., GEISLER, R., et
al. (2002). Retinoic acid signalling in the zebrafish embryo is necessary during pre-segmentation stages to pattern the anterior-posterior axis of the CNS and to induce a pectoral fin bud. Development 129: 2851-2865.
HAECKEL, E. (1874). Anthropogenie oder Entwickelungsgeschichte des Menschen. (Leipzig: Engelmann).
HIIRAGI, T. AND SOLTER, D. (2004). First cleavage plane of the mouse egg is not predetermined but defined by the topology of the two apposing pronuclei. Nature 430: 360-364.
HOOIVELD, M. H., MORGAN, R., IN DER RIEDEN, P., HOUTZAGER, E., PANNESE,
M., DAMEN, K., BONCINELLI, E. AND DURSTON, A. J. (1999). Novel interactions
between vertebrate Hox genes. Int J Dev Biol 43: 665-674.
HORAN, G. S., RAMIREZ-SOLIS, R., FEATHERSTONE, M. S., WOLGEMUTH, D.
J., BRADLEY, A. AND BEHRINGER, R. R. (1995). Compound mutants for the
paralogous hoxa-4, hoxb-4 and hoxd-4 genes show more complete homeotic
transformations and a dose-dependent increase in the number of vertebrae
transformed. Genes Dev 9: 1667-1677.
HOUART, C., CANEPARO, L., HEISENBERG, C., BARTH, K., TAKE-UCHI, M.
AND WILSON, S. (2002). Establishment of the telencephalon during gastrulation by local antagonism of Wnt signaling. Neuron 35: 255-265.
HUANG, D., CHEN, S. W., LANGSTON, A. W. AND GUDAS, L. J. (1998). A
conserved retinoic acid responsive element in the murine Hoxb-1 gene is
required for expression in the developing gut. Development 125: 3235-3246.
HUYNH, J. R. AND ST JOHNSTON, D. (2004). The origin of asymmetry: early
polarisation of the Drosophila germline cyst and oocyte. Curr Biol 14: R438-449.
INOUE, T., NAKAMURA, S. AND OSUMI, N. (2000). Fate mapping of the mouse
prosencephalic neural plate. Dev Biol 219: 373-383.
ISAACS, H. V., POWNALL, M. E. AND SLACK, J. M. (1998). Regulation of Hox gene expression and posterior development by the Xenopus caudal homologue Xcad3. EMBO J 17: 3413-3427.
ITASAKI: N., ICHIJO, H., HAMA, C., MATSUNO, T. AND NAKAMURA, H. (1991).
Establishment of rostrocaudal polarity in tectal primordium: engrailed expression and subsequent tectal polarity. Development 113: 1133-1144.
ITASAKI, N., JONES, C. M., MERCURIO, S., ROWE, A., DOMINGOS, P. M.,
SMITH, J. C. AND KRUMLAUF, R. (2003). Wise, a context-dependent activator
and inhibitor of Wnt signalling. Development 130: 4295-4305.
KANE, D. A. AND WARGA, R. M. (2004). Teleost gastrulation, In Gastrulation: from cells to embryo, C. D. Stern, ed. (New York: Cold Spring Harbor Press), pp. 157-169.
KELLER, R. AND SHOOK, D. (2004). Gastrulation in amphibians, In Gastrulation: from cells to embryo, C. D. Stern, ed. (New York: Cold Spring Harbor Press), pp. 171-204.
KESSEL, M. (1992). Respecification of vertebral identities by retinoic acid. Development 115: 487-501.
KESSEL, M. AND GRUSS, P. (1991). Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell 67: 89-104.
KINDER, S. J., TSANG, T. E., QUINLAN, G. A., HADJANTONAKIS, A. K., NAGY, A. AND TAM, P. P. (1999). The orderly allocation of mesodermal cells to the extraembryonic structures and the anteroposterior axis during gastrulation of the mouse embryo. Development 126: 4691-4701.
KMITA, M. AND DUBOULE, D. (2003). Organizing axes in time and space; 25 years of colinear tinkering. Science 301: 331-333.
KNOETGEN, H., VIEBAHN, C. AND KESSEL, M. (1999). Head induction in the
chick by primitive endoderm of mammalian, but not avian origin. Development
126: 815-825.
KOCHAV, S. AND EYAL-GILADI, H. (1971). Bilateral symmetry in chick embryo determination by gravity. Science 171: 1027-1029.
KUAN, C. Y., TANNAHILL, D., COOK, G. M. AND KEYNES, R. J. (2004). Somite polarity and segmental patterning of the peripheral nervous system. Mech Dev 121: 1055-1068.
KUDOH, T., CONCHA, M. L., HOUART, C., DAWID, I. B. AND WILSON, S. W.
(2004). Combinatorial Fgf and Bmp signalling patterns the gastrula ectoderm
into prospective neural and epidermal domains. Development 131: 3581-3592.
KUDOH, T., WILSON, S. W. AND DAWID, I. B. (2002). Distinct roles for Fgf, Wnt and retinoic acid in posteriorizing the neural ectoderm. Development 129: 4335-4346.
LALL, S. AND PATEL, N. H. (2001). Conservation and divergence in molecular mechanisms of axis formation. Annu Rev Genet 35: 407-437.
LANE, M. C. AND SHEETS, M. D. (2000). Designation of the anterior/posterior axis in pregastrula Xenopus laevis. Dev Biol 225: 37-58.
LAWSON, K. A., MENESES, J. J. AND PEDERSEN, R. A. (1991). Clonal analysis of epiblast fate during germ layer formation in the mouse embryo. Development 113: 891-911.
LIU, J. P., LAUFER, E. AND JESSELL, T. M. (2001). Assigning the positional identity of spinal motor neurons: rostrocaudal patterning of Hox-c expression by FGFs, Gdf11 and retinoids. Neuron 32: 997-1012.
LU, C. C., BRENNAN, J. AND ROBERTSON, E. J. (2001). From fertilization to gastrulation: axis formation in the mouse embryo. Curr Opin Genet Dev 11: 384-392.
LUMSDEN, A. (2004). Segmentation and compartition in the early avian hindbrain.Mech Dev 121: 1081-1088.
LUMSDEN, A. AND KEYNES, R. (1989). Segmental patterns of neuronal development in the chick hindbrain. Nature 337: 424-428.
MAINGUY, G., IN DER RIEDEN, P. M., BEREZIKOV, E., WOLTERING, J. M.,
PLASTERK, R. H. AND DURSTON, A. J. (2003). A position-dependent
organisation of retinoid response elements is conserved in the vertebrate Hox clusters. Trends Genet 19: 476-479.
MANGOLD, O. (1933). Ьber die Induktionsfдhighkeit der verschiedenen Bezirke der Neurula von Urodelen. Naturwissenshaften 21: 761-766.
MARSHALL, H., NONCHEV, S., SHAM, M. H., MUCHAMORE, I., LUMSDEN, A.
AND KRUMLAUF, R. (1992). Retinoic acid alters hindbrain Hox code and
induces transformation of rhombomeres 2/3 into a 4/5 identity. Nature 360: 737-741.
MARTINEZ, S. AND ALVARADO-MALLART, R. M. (1990). Expression of the
homeobox Chick-en gene in chick/quail chimeras with inverted mes-metencephalic grafts. Dev Biol 139: 432-436.
MARTINEZ, S., MARIN, F., NIETO, M. A. AND PUELLES, L. (1995). Induction of ectopic engrailed expression and fate change in avian rhombomeres: intersegmental boundaries as barriers. Mech Dev 51: 289-303.
MATHIS, L. AND NICOLAS, J. F. (2000). Different clonal dispersion in the rostral and caudal mouse central nervous system. Development 127: 1277-1290.
MAVILIO, F., SIMEONE, A., BONCINELLI, E. AND ANDREWS, P. W. (1988).
Activation of four homeobox gene clusters in human embryonal carcinoma cells induced to differentiate by retinoic acid. Differentiation 37: 73-79.
MCGINNIS, W. AND KRUMLAUF, R. (1992). Homeobox genes and axial patterning. Cell 68: 283-302.
MCGREW, L. L., HOPPLER, S. AND MOON, R. T. (1997). Wnt and FGF pathways
cooperatively pattern anteroposterior neural ectoderm in Xenopus. Mech Dev
69: 105-114.
MESSENGER, N. J., KABITSCHKE, C. ANDREWS, R., GRIMMER, D., MIGUEL,
R. N., BLUNDELL, T. L., SMITH, J. C. AND WARDLE, F. C. (2005). Functional
specificity of the Xenopus T-domain protein brachyury is conferred by its ability to interact with smad1. Dev Cell 8: 599-610.
MOLOTKOVA, N., MOLOTKOV, A., SIRBU, I. O. AND DUESTER, G. (2005).
Requirement of mesodermal retinoic acid generated by Raldh2 for posterior
neural transformation. Mech Dev 122: 145-155.
MORENO, T. A. AND KINTNER, C. (2004). Regulation of segmental patterning by retinoic acid signaling during Xenopus somitogenesis. Dev Cell 6: 205-218.
MORKEL, M., HUELSKEN, J., WAKAMIYA, M., DING, J., VAN DE WETERING, M.,
CLEVERS, H., TAKETO, M. M., BEHRINGER, R. R., SHEN, M. M. AND
BIRCHMEIER, W. (2003). Beta-catenin regulates Cripto- and Wnt3-dependent
gene expression programs in mouse axis and mesoderm formation. Development
130: 6283-6294.
MUHR, J., GRAZIANO, E., WILSON, S., JESSELL, T. M. AND EDLUND, T. (1999). Convergent inductive signals specify midbrain, hindbrain and spinal cord identity in gastrula stage chick embryos. Neuron 23: 689-702.
MUHR, J., JESSELL, T. M. AND EDLUND, T. (1997). Assignment of early caudal identity to neural plate cells by a signal from caudal paraxial mesoderm. Neuron 19: 487-502.
NICOLAS, J. F., MATHIS, L., BONNEROT, C. AND SAURIN, W. (1996). Evidence in the mouse for self-renewing stem cells in the formation of a segmented longitudinal structure, the myotome. Development 122: 2933-2946.
NIEUWKOOP, P. D., BOTTERNENBROOD, E. C., KREMER, A., BLOESMA, F. F.
S. N., HOESSELS, E. L. M. J., MEYER, G. AND VERHEYEN, F. J. (1952).
Activation and organization of the Central Nervous System in Amphibians. J Exp Zool 120: 1-108.
NIEUWKOOP, P. D. AND NIGTEVECHT, G. V. (1954). Neural activation and
transformation in explants of competent ectoderm under the influence of
fragments of anterior notochord in urodeles. J Embryol Exp Morphol 2: 175-193.
OOSTERVEEN, T., NIEDERREITHER, K., DOLLE, P., CHAMBON, P., MEIJLINK,
F. AND DESCHAMPS, J. (2003). Retinoids regulate the anterior expression
boundaries of 5' Hoxb genes in posterior hindbrain. EMBO J 22: 262-269.
PACKER, A. I., CROTTY, D. A., ELWELL, V. A. AND WOLGEMUTH, D. J. (1998). Expression of the murine Hoxa4 gene requires both autoregulation and a conserved retinoic acid response element. Development 125: 1991-1998.
PALMEIRIM, I., HENRIQUE, D., ISH-HOROWICZ, D. AND POURQUIE, O. (1997).
Avian hairy gene expression identifies a molecular clock linked to vertebrate segmentation and somitogenesis. Cell 91: 639-648.
PERA, E. M. AND KESSEL, M. (1997). Patterning of the chick forebrain anlage by the prechordal plate. Development 124: 4153-4162.
PEREA-GOMEZ, A., VELLA, F. D., SHAWLOT, W., OULAD-ABDELGHANI, M.,
CHAZAUD, C., MENO, C., PFISTER, V., CHEN, L., ROBERTSON, E., HAMADA,
H., et al. (2002). Nodal antagonists in the anterior visceral endoderm prevent the formation of multiple primitive streaks. Dev Cell 3: 745-756.
PFEFFER, P. L. AND DE ROBERTIS, E. M. (1994). Regional specificity of RAR gamma isoforms in Xenopus development. Mech Dev 45: 147-153.
PLUSA, B., HADJANTONAKIS, A. K., GRAY, D., PIOTROWSKA-NITSCHE, K.,
JEDRUSIK, A., PAPAIOANNOU, V. E., GLOVER, D. M. AND ZERNICKAGOETZ,
M. (2005). The first cleavage of the mouse zygote predicts the
blastocyst axis. Nature 434: 391-395.
POURQUIE, O. (2004). The chick embryo: a leading model in somitogenesis
studies. Mech Dev 121: 1069-1079.
POWNALL, M. E., ISAACS, H. V. AND SLACK, J. M. (1998). Two phases of Hox gene regulation during early Xenopus development. Curr Biol 8: 673-676.
POWNALL, M. E., TUCKER, A. S., SLACK, J. M. AND ISAACS, H. V. (1996). eFGF, Xcad3 and Hox genes form a molecular pathway that establishes the anteroposterior axis in Xenopus. Development 122: 3881-3892.
PRIMMETT, D. R., NORRIS, W. E., CARLSON, G. J., KEYNES, R. J. AND STERN, C. D. (1989). Periodic segmental anomalies induced by heat shock in the chick embryo are associated with the cell cycle. Development 105: 119-130.
PRINCE, V. E., PRICE, A. L. AND HO, R. K. (1998). Hox gene expression reveals regionalization along the anteroposterior axis of the zebrafish notochord. Dev Genes Evol 208: 517-522.
PSYCHOYOS, D. AND STERN, C. D. (1996). Fates and migratory routes of
primitive streak cells in the chick embryo. Development 122: 1523-1534.
PUELLES, L. AND RUBENSTEIN, J. L. (1993). Expression patterns of homeobox and other putative regulatory genes in the embryonic mouse forebrain suggest a neuromeric organization. Trends Neurosci 16: 472-479.
PUELLES, L. AND RUBENSTEIN, J. L. (2003). Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci 26: 469-476.
RICHARDSON, M. K. AND KEUCK, G. (2002). Haeckel’s ABC of evolution and
development. Biol Rev Camb Philos Soc 77: 495-528.
ROBERTSON, E. J., NORRIS, D. P., BRENNAN, J. AND BIKOFF, E. K. (2003).
Control of early anterior-posterior patterning in the mouse embryo by TGF-beta signalling. Philos Trans R Soc Lond B Biol Sci 358: 1351-1357.
ROELEN, B. A., DE GRAAFF, W., FORLANI, S. AND DESCHAMPS, J. (2002). Hox
cluster polarity in early transcriptional availability: a high order regulatory level of clustered Hox genes in the mouse. Mech Dev 119: 81-90.
RUIZ I ALTABA, A. AND JESSELL, T. M. (1991). Retinoic acid modifies the pattern of cell differentiation in the central nervous system of neurula stage Xenopus embryos. Development 112: 945-958.
SELLECK, M. A. AND STERN, C. D. (1991). Fate mapping and cell lineage analysis of Hensen’s node in the chick embryo. Development 112: 615-626.
SELLECK, M. A. J. AND STERN, C. D. (1992). Evidence for stem cells in the mesoderm of Hensen’s node and their role in embryonic pattern formation., In Formation and differentiation of early embryonic mesoderm., R. Bellairs, E. J. Sanders and J. W. Lash, eds. (New York: Plenum Press), pp. 23-31.
SHIMAMURA, K. AND RUBENSTEIN, J. L. (1997). Inductive interactions direct early regionalization of the mouse forebrain. Development 124: 2709-2718.
SHIOTSUGU, J., KATSUYAMA, Y., ARIMA, K., BAXTER, A., KOIDE, T., SONG, J., CHANDRARATNA, R. A. AND BLUMBERG, B. (2004). Multiple points of
interaction between retinoic acid and FGF signaling during embryonic axis
formation. Development 131: 2653-2667.
SIMEONE, A. (2000). Positioning the isthmic organizer where Otx2 and Gbx2meet. Trends Genet 16: 237-240.
SIMEONE, A., ACAMPORA, D., ARCIONI, L. ANDREWS, P. W., BONCINELLI, E.
AND MAVILIO, F. (1990). Sequential activation of HOX2 homeobox genes by
retinoic acid in human embryonal carcinoma cells. Nature 346: 763-766.
SIMEONE, A., ACAMPORA, D., NIGRO, V., FAIELLA, A., D’ESPOSITO, M.,
STORNAIUOLO, A., MAVILIO, F. AND BONCINELLI, E. (1991). Differential
regulation by retinoic acid of the homeobox genes of the four HOX loci in human embryonal carcinoma cells. Mech Dev 33: 215-227.
SOCKANATHAN, S., PERLMANN, T. AND JESSELL, T. M. (2003). Retinoid
receptor signaling in postmitotic motor neurons regulates rostrocaudal positional identity and axonal projection pattern. Neuron 40: 97-111.
SOLNICA-KREZEL, L. (2005). Conserved Patterns of Cell Movements during
Vertebrate Gastrulation. Curr Biol 15: R213-R228.
SPEMANN, H. AND MANGOLD, H. (1924). Induction of embryonic primordia by
implantation of organizers from a different species. Roux’ Arch EntwMech Org 100: 599-638. Re-edition of Viktor Hamburgerґs translation of the original 1924 paper entitled Ьber Induktion von Embryonalanlagen durch Implantation artfremder Organisatoren Int. J. Dev. Biol. 45: 13-38 (2001)
SPRATT, N. T. AND HAAS, H. (1960). Integrative mechanisms in development of the early chick blastoderm. I. Regulative potentiality of separated parts. J Exp Zool 145: 97-137.
STERN, C. D. (1990). Two distinct mechanisms for segmentation? Semin Dev Biol 1: 109-116.
STERN, C. D. (2001). Initial patterning of the central nervous system: how many organizers? Nat Rev Neurosci 2: 92-98.
STERN, C. D. (2004). Gastrulation in the chick, In Gastrulation: from cells to embryo, C. D. Stern, ed. (New York: Cold Spring Harbor Press), pp. 219-232.
STERN, C. D., FRASER, S. E., KEYNES, R. J. AND PRIMMETT, D. R. (1988). A cell lineage analysis of segmentation in the chick embryo. Development 104 Suppl: 231-244.
STERN, C. D., HATADA, Y., SELLECK, M. A. AND STOREY, K. G. (1992).
Relationships between mesoderm induction and the embryonic axes in chick
and frog embryos. Development Suppl: 151-156.
STOREY, K. G., GORIELY, A., SARGENT, C. M., BROWN, J. M., BURNS, H. D.,
ABUD, H. M. AND HEATH, J. K. (1998). Early posterior neural tissue is induced by FGF in the chick embryo. Development 125: 473-484.
TAM, P. P. AND STEINER, K. A. (1999). Anterior patterning by synergistic activity of the early gastrula organizer and the anterior germ layer tissues of the mouse embryo. Development 126: 5171-5179.
TAM, P. P. L. AND GAD, J. L. (2004). Gastrulation in the mouse embryo, In Gastrulation: from cells to embryo, C. D. Stern, ed. (New York: Cold Spring Harbor Press), pp. 233-262.
TAUTZ, D. (2004). Segmentation. Dev Cell 7: 301-312.
THOMAS, P. AND BEDDINGTON, R. (1996). Anterior primitive endoderm may be
responsible for patterning the anterior neural plate in the mouse embryo. Curr Biol 6: 1487-1496.
VAAGE, S. (1969). The segmentation of the primitive neural tube in chick embryos (Gallus domesticus). A morphological, histochemical and autoradiographical investigation. Ergeb Anat Entwicklungsgesch 41: 3-87.
VAN DEN AKKER, E., FORLANI, S., CHAWENGSAKSOPHAK, K., DE GRAAFF,
W., BECK, F., MEYER, B. I. AND DESCHAMPS, J. (2002). Cdx1 and Cdx2 have
overlapping functions in anteroposterior patterning and posterior axis elongation. Development 129: 2181-2193.
WACKER, S. A., JANSEN, H. J., MCNULTY, C. L., HOUTZAGER, E. AND
DURSTON, A. J. (2004a). Timed interactions between the Hox expressing nonorganiser mesoderm and the Spemann organiser generate positional information during vertebrate gastrulation. Dev Biol 268: 207-219.
WACKER, S. A., MCNULTY, C. L. AND DURSTON, A. J. (2004b). The initiation of Hox gene expression in Xenopus laevis is controlled by Brachyury and BMP-4. Dev Biol 266: 123-137.
WILSON, S. W. AND HOUART, C. (2004). Early steps in the development of the forebrain. Dev Cell 6: 167-181.
WITHINGTON, S., BEDDINGTON, R. AND COOKE, J. (2001). Foregut endoderm
is required at head process stages for anteriormost neural patterning in chick.Development 128: 309-320.
WOO, K. AND FRASER, S. E. (1995). Order and coherence in the fate map of the zebrafish nervous system. Development 121: 2595-2609.
WURST, W. AND BALLY-CUIF, L. (2001). Neural plate patterning: upstream and
downstream of the isthmic organizer. Nat Rev Neurosci 2: 99-108.
ZAKANY, J., GERARD, M., FAVIER, B. AND DUBOULE, D. (1997). Deletion of a HoxD enhancer induces transcriptional heterochrony leading to transposition of the sacrum. EMBO J 16: 4393-4402.
ZAKANY, J., KMITA, M., ALARCON, P., DE LA POMPA, J. L. AND DUBOULE, D.
(2001). Localized and transient transcription of Hox genes suggests a link
between patterning and the segmentation clock. Cell 106: 207-217.
Jr-Kai Yu, Yutaka Satou, Nicholas D. Holland, Tadasu Shin-I, Yuji Kohara, Noriyuki Satoh, Marianne Bronner-Fraser and Linda Z. Holland
Axial patterning in cephalochordates and the evolution of the organizer
Nature 445, 613-617 (8 February 2007) | doi:10.1038/nature05472;
Организатор гаструлы у позвоночных является важным сигнальным центром, который индуцирует и формирует паттерн дорсальных осевых структур. Несмотря на длительный интерес эволюционное происхождение организатора остается неясным. В данной работе было показано, что гаструла головохордовых (cephalochordate) amphioxus экспрессирует гены для дорсо/вентрального формирования паттерна (напр., bone morphogenetic proteins (BMPs), Nodal и их антагонисты) по способу, напоминающему экспрессию соотв. ортологов у позвоночных , и что эмбрионы amphioxus, подобно эмбрионам позвоночных вентрализуются с помощью BMP белка. Кроме того, Wnt-антагонисты (напр., Dkks и sFRP2-like) экспрессируются в передних частях, тогда как сами Wnt гены экспрессируются более кзади, это согласуется с ролью передачи сигналов Wnt для формирования передне/заднего паттерна. Эти результаты подтверждают эволюционную консервацию механизмов формирования как D/V , так и A/P паттерна ранней гаструлы. В свете недавнего филогенетического анализа, поместившего cephalochordates в основном в линиию хордовых, мы предполагаем, что отдельные сигнальные центры для формирования D/V и A/P осей могут быть родоначальными характеритиками хордовых.
| When, where and how is the head-tail axis of the embryo set
up during development? These are such fundamental and intensely
studied questions that one might expect them to have
been answered long ago. Not so; we still understand very little
about the cellular or molecular mechanisms that lead to the
orderly arrangement of body elements along the head-tail axis in
vertebrates. In this paper, we outline some of the major outstanding
problems and controversies and try to identify some reasons
why it has been so difficult to resolve this important issue.
|
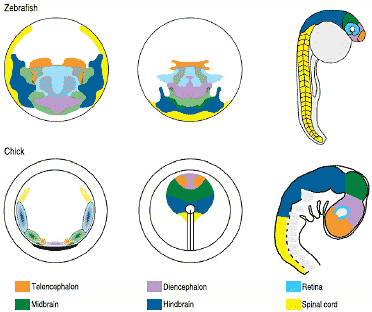 Fig. 1. Rough fate maps of zebrafish (upper row) and chick (lower row) embryos at early blastula (left), gastrula (middle) and a stage when the nervous system has been roughly patterned (right). The earliest stage chick embryo displays predictions of the locations of the centres of these prospective territories since the regions overlap considerably at this stage. Based on data from Woo and Fraser (1995), Hatada and Stern (1994) and various fate maps from the literature.
Fig. 1. Rough fate maps of zebrafish (upper row) and chick (lower row) embryos at early blastula (left), gastrula (middle) and a stage when the nervous system has been roughly patterned (right). The earliest stage chick embryo displays predictions of the locations of the centres of these prospective territories since the regions overlap considerably at this stage. Based on data from Woo and Fraser (1995), Hatada and Stern (1994) and various fate maps from the literature. 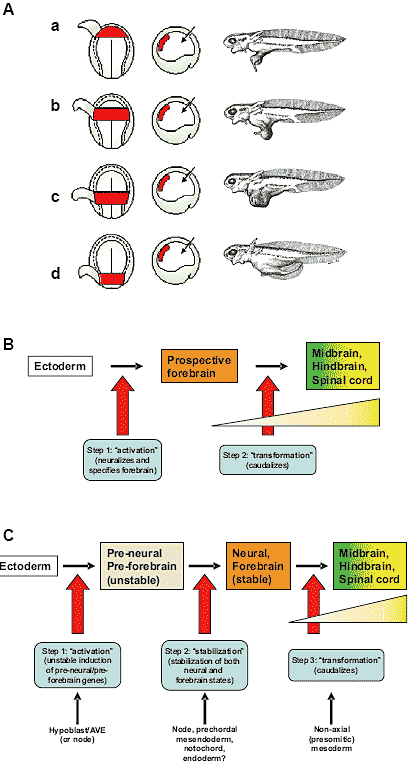 Fig. 2. Three models to explain initial head-tail patterning of the embryo. (A) The "head/trunk/tail organizer" model, based on the experiments of Otto Mangold (shown schematically in the first two columns, with the result on the right): grafts of "anterior"
archenteron roof induce an ectopic head, those of mid-level roof induce trunk and those of caudalmost roof tissue induce a tail. (B) Nieuwkoop's "activation-transformation" model. (C) The "three-step" model, a modification of Nieuwkoop's model.
Fig. 2. Three models to explain initial head-tail patterning of the embryo. (A) The "head/trunk/tail organizer" model, based on the experiments of Otto Mangold (shown schematically in the first two columns, with the result on the right): grafts of "anterior"
archenteron roof induce an ectopic head, those of mid-level roof induce trunk and those of caudalmost roof tissue induce a tail. (B) Nieuwkoop's "activation-transformation" model. (C) The "three-step" model, a modification of Nieuwkoop's model. 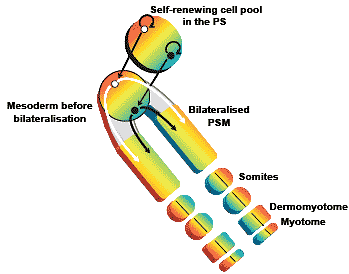 Fig. 3. Stem-cell-like cells resident in Hensen's node (circle on upper left of diagram) divide within the node. At each division, one daughter remains in the node while the other and its subseuqent progeny colonizes the paraxial (prospective somite) mesoderm (shown as bilateral rods extending towards the lower right of the diagram). From Eloy-Trinquet et al.(2002), in turn based on data from Selleck and Stern (1991, 1992) and
Nicolas et al. (1996).
Fig. 3. Stem-cell-like cells resident in Hensen's node (circle on upper left of diagram) divide within the node. At each division, one daughter remains in the node while the other and its subseuqent progeny colonizes the paraxial (prospective somite) mesoderm (shown as bilateral rods extending towards the lower right of the diagram). From Eloy-Trinquet et al.(2002), in turn based on data from Selleck and Stern (1991, 1992) and
Nicolas et al. (1996). 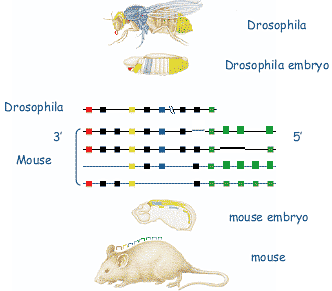 Fig. 4. Chromosomal organization of a Hox cluster and schematic
expression pattern in Drosophila (upper) and mouse (lower). The fly
has only one Hox cluster, while the mouse has four. Hox clusters are
spatially and temporally colinear: genes situated towards the 3' end of the
cluster (shown on the left in the diagrams) are expressed more rostrally and earlier than those closer to the 5' end.
Fig. 4. Chromosomal organization of a Hox cluster and schematic
expression pattern in Drosophila (upper) and mouse (lower). The fly
has only one Hox cluster, while the mouse has four. Hox clusters are
spatially and temporally colinear: genes situated towards the 3' end of the
cluster (shown on the left in the diagrams) are expressed more rostrally and earlier than those closer to the 5' end.