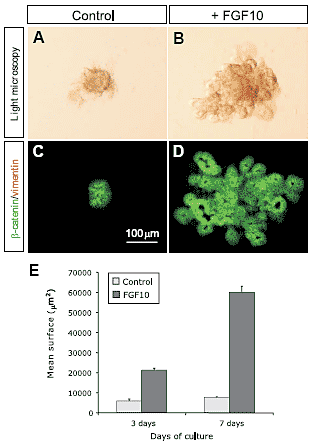
 Fig. 1. Effects of FGF10 on the growth and morphogenesis of isolated E10.5 dorsal pancreatic epithelia. (A,B) Isolated pancreatic epithelia grown in vitro for 7 days, without and with 100 ng/ml FGF10. (A) The untreated epithelium fails to grow and remains as a dense agregate of cells with occasional budding. (B) After 7 days of culture, the FGF10-treated epithelium shows an irregular structure formed by buds, cysts and tubules. (C,D) в-catenin/vimentin staining of the pancreatic epithelium after 7 days of culture. (E) Quantitative analysis of the size of the isolated pancreatic epithelia after 3 and 7 days of culture. The bars represent the mean ± SEM of 6 epithelia.
Fig. 1. Effects of FGF10 on the growth and morphogenesis of isolated E10.5 dorsal pancreatic epithelia. (A,B) Isolated pancreatic epithelia grown in vitro for 7 days, without and with 100 ng/ml FGF10. (A) The untreated epithelium fails to grow and remains as a dense agregate of cells with occasional budding. (B) After 7 days of culture, the FGF10-treated epithelium shows an irregular structure formed by buds, cysts and tubules. (C,D) в-catenin/vimentin staining of the pancreatic epithelium after 7 days of culture. (E) Quantitative analysis of the size of the isolated pancreatic epithelia after 3 and 7 days of culture. The bars represent the mean ± SEM of 6 epithelia.
Discussion
To study the effects of FGF10 on the self-renewal of the
pancreatic progenitors we have used explant cultures of isolated
E10.5 mouse dorsal pancreatic epithelia. The explants were
grown into reduced growth factors Matrigel with or without FGF10
and their development analyzed after three or seven days of
culture. Our results showed that after three days of culture the
untreated pancreatic epithelia did not increase in size while those
cultured with FGF10 presented a 4-fold increase in size. The
analysis of BrdU incorporation indicated very low proliferation in
the untreated rudiments and active proliferation in those receiving
FGF10. The untreated pancreatic epithelia were essentially composed
by endocrine cells (90% of the total cell surface in the
rudiment) and only a few cells remained undifferentiated (10%).
In the FGF10 treated epithelia the reverse was observed: the
endocrine cells represented only 15% of the rudiment surface
area while the remaining 85% was composed of undifferentiated
cells. Our results indicate that in the untreated pancreatic epithelia
the progenitors cells fail to proliferate and differentiate rapidly.
In contrast, in the FGF10-treated epithelia the pancreatic progenitors
proliferate actively and remain in an undifferentiated state
characterized by the co-expression of the transcription factors
Pdx1, Nkx6.1, Ptf1б and Hes1.
After seven days of culture the untreated pancreatic epithelia
did not increase in size and were composed essentially by
endocrine cells (85%) and a few acinar cells (15%). On the
contrary during this period the explants treated with FGF10
underwent a 10-fold increase in size. In these rudiments the
endocrine cells represented only 10% of the total cell surface,
most cells being acinar cells (40%) or undifferentiated cells
(50%). Interestingly BrdU incorporation in these explants concerned
always the acinar cells (15%) and the undifferentiated
cells (20%). Taken together these results indicate that FGF10 has
different effects on the proliferation of the pancreatic epithelia. It
stimulates the proliferation of pancreatic progenitors and induces
the proliferation of differentiated acinar cells. The high BrdU
labelling index of the acinar cells in the FGF10 treated rudiments
suggests that most of these cells arise by proliferation
of the few acinar cells which differentiate
from the isolated pancreatic epithelia. It is well
established that acinar differentiation is dependent
on mesenchymal signals (Rutter et al., 1978;
Gittes et al., 1996; Miralles et al., 1998). However,
in our culture conditions we observed that
a few acinar cells form in isolated pancreatic
epithelium. The absence of vimentin staining
indicated that there was no contamination by
mesechyme cells in these cultures. Therefore
these cells probably arise from pancreatic progenitors,
which were already engaged in acinar
differentiation prior to mesenchyme removal. In
this respect it is noteworthy that although not
detectable by immunohistochemistry, some specific
acinar products like carboxypeptidase A can
be detected by RT-PCR as early as E9.5 in the
pancreatic rudiments of mice (Gittes and Rutter,
1992), (and our own results). An alternative explanation
would be that the role of the mesenchyme
in pancreatic development is to furnish
permissive rather than instructive signals. The
FGF10 (as well as other growth factors) secreted
by the mesenchyme would allow the expansion
of progenitor cells, that subsequently differentiate
into exocrine cells.
The trophic effects of FGF10 on the mouse
pancreatic epithelia were not unexpected since
we had previously shown that FGF10 stimulates
the proliferation of the rat embryonic pancreas
(Miralles et al., 1999). Moreover FGF10-null mice
presented an hypoplastic pancreas (Bhushan et al., 2001), while transgenic mice over expressing FGF10 in the
pancreas displayed pancreatic hyperplasia (Hart et al.,
2003;Norgaard et al., 2003). It should be mentioned that transgenic
expression of a dominant negative FGFR2b under the
control of the Pdx1 promoter did not lead to a pancreatic phenotype
(Hart et al., 2000) and only a week pancreatic hypoplasia was
observed in the FGFR2b-null mice (Pulkkinen et al., 2003). These
studies seem to contradict the hypothesis that FGF10 could play
a major role in the control of the proliferation of the undifferentiated
pancreatic epithelia. However it must be noted that FGF10
might signal trough other receptors (Powers et al., 2000). Moreover,
other factors like FGF2, FGF7, EGF and HGF are also able
to stimulate the proliferation of the embryonic pancreatic epithelia
and could compensate the loss of FGF10 signalling (Kim and
MacDonald, 2002;Edlund, 2002).
Another important effect of FGF10 is its capacity to maintain a
considerable number of cells in an undifferentiated state. As
indicated above, after seven days of culture in the presence of
FGF10 a considerable number of cells in the isolated pancreatic
epithelia did not stain positively for endocrine or exocrine markers
of pancreatic differentiation. These cells co-expressed the transcription
factors Pdx1, Nkx6.1 and p48/Ptf1б, which is a characteristic
of pancreatic progenitors. Moreover, most of the undifferentiated
cells in the FGF10-treated epithelia express the transcription
factor Hes1. This is another characteristic of pancreatic
progenitors and indicates that the Notch pathway is active in these
cells. These results are similar to what has been reported in the
pPdx1-FGF10 transgenic mice, which showed pancreatic hyperplasia,
maintenance of pancreatic progenitors in an undifferentiated
state and persistent Notch activation (Hart et al.,
2003;Norgaard et al., 2003). In our in vitro cultures, acinar
differentiation was not so efficiently blocked as it was in the
transgenic mice. This could be explained by the fact that in the
transgenic mice FGF10 is expressed at the onset of Pdx1 expression,
that is E8.5. Thus, in these animals, FGF10 is acting on the
early pancreatic progenitors. In our study we have used E10.5
pancreatic epithelium. At this stage most cells in the pancreatic
rudiment are progenitor cells, but a few cells have differentiated
into endocrine cells and others are probably, as mentioned above,
already engaged into acinar differentiation. The mitotic effect of
FGF10 on the few acinar cells which differentiate spontaneously
probably enhances the relative proportion of acinar cells in our
model.
The proportion of undifferentiated cells in the mouse explants
is increased comparatively to what was previously observed
using isolated E11.5 rat dorsal pancreatic epithelium (Miralles et
al., 1999). The E11.5 rat and the E10.5 mouse dorsal pancreatic
rudiments are very similar in terms of transcription factors expression
and cell differentiation status. Thus, the differences are
essentially due to different culture conditions. The cultures were
done in serum free conditions in previous studies whereas we
added 1% fetal calf serum (FCS) in the present study. We noted that despite the presence of FGF10, a minimal amount of other
growth factors was necessary to allow the survival and growth of
the pancreatic precursors when cultured in the absence of mesenchyme.
Another unexpected observation is the absence of ductal cells
in our cultures. Gittes and co-workers have shown that isolated
mouse pancreatic epithelium grown in Matrigel developed into
cystic structures formed by differentiated ductal cells (Gittes et al.,
1996). In our study we have used reduced growth factor Matrigel,
which contains the same basement membrane components than
Matrigel but has been greatly depleted in growth factors. Apparently
our culture conditions are less favourable to ductal differentiation.
However, a few ductal cells were detected in the explants
treated simultaneously with FGF10 and compound 1 suggesting
that somehow, the blockage of Notch signalling could be required
to allow ductal differentiation. It must be noted also that, as in the
FGF10-treated explants, differentiated ductal cells were not detected
in the pPdx1-FGF10 transgenic mice.
The role of the Notch pathway in the control of pancreatic
differentiation is now well established. Loss of function of various
Notch pathway genes (Hes1, Delta1, RBPjk) leads to premature
and massive differentiation of the pancreatic progenitors into
endocrine cells (Apelqvist et al., 1999;Gradwohl et al., 2000;Jensen
et al., 2000). A similar phenotype was observed in transgenic
mice expressing the bHLHL trascription factor Ngn3 (a gene
usually repressed by Notch activation) under the control of the Pdx1 promoter ( pPdx1-Ngn3 ), (Apelqvist et al., 1999).
Moreover, when a Notch-IC transgene is activated in the
developing mouse pancreas using the Pdx1 promoter,
both endocrine and exocrine differentiation are repressed,
suggesting that Notch has an inhibitory role in the control
of the differentiation of both lineages (Murtaugh et al.,
2003). It has been suggested that maintenance of the
pancreatic progenitors in an undifferentiated state in the
pPdx1-FGF10 mice could result of an eventual effect of
FGF10 in inducing persistent activation of the Notch pathway.
Our study corroborates this hypothesis. Hes1, a
target gene of the Notch pathway, was rapidly downregulated
in the isolated E10.5 pancreatic epithelia, but its
expression persisted in the explants treated with FGF10.
Moreover, the г-secretase inhibitor, compound 1, downregulated
Hes1 expression and considerably reduced the
growth and the number of undifferentiated cells in the
FGF10-treated pancreatic epithelia. Thus, the inhibition of
the Notch pathway prevents the effect of FGF10 on the
proliferation and the maintenance of the pancreatic progenitors
in an undifferentiated state. Therefore, the Notch
pathway is required as a downstream mediator of the
FGF10 signalling in pancreatic precursors. We do not
know how FGF10 maintains the Notch activation. It has
been suggested, based on the expression of the Notch
ligand genes Jagged 1 and Jagged 2 in the undifferentiated
pancreatic epithelium of the pPdx1-FGF10 mice, that
FGF10 could induce the expression of these ligands
(Norgaard et al., 2003). FGF10 could also down-regulate
repressors of Notch activity like Sel1 (Hart et al., 2003) or
up regulate Notch expression. In this regard, it has been
shown that the FGF1 and FGF2 induce the proliferation
and inhibit the differentiation of neuroepithelial precursors trough Notch signalling. Both FGFs efficiently up-regulate the
expression of Notch 1 in these neuronal precursors (Faux et al.,
2001). However, our RT-PCR analysis did not show any major
differences in the levels of expression of these genes between the
FGF10-treated and untreated pancreatic epithelia.
It is noteworthy that untreated isolated pancreatic epithelia
showed in vitro an outcome similar to that of the pancreas of mice
deficient for different genes of the Notch pathway, or the pPdx1-
Ngn3 mice. That is, arrested growth, accelerated and almost total
differentiation of the pancreatic epithelium into endocrine cells
and also, as we observed in our study, rapid down-regulation of
Hes1. This suggests that the mesenchyme not only provides
signals necessary for the growth of the pancreatic epithelium but
it can also regulate the maintenance of the pancreatic progenitors
in an undifferentiated state via the Notch pathway. In the developing
pancreas FGF10 is essentially produced by the pancreatic
mesenchyme, while its receptor FGFR2b is expressed only in the
epithelial cells (Miralles et al., 1999). Thus, the pattern of expression
of FGF10 is consistent with the hypothesis that FGF10 could
be the mesenchymal factor responsible of the maintenance of the
Notch signalling. Studies on the development of other organs
have also implicated the FGFs in the self-renewal of progenitor
cells via the Notch pathway. Of particular interest, in this context,
are several studies showing that FGF10 is capable of preventing
the differentiation of the odontoblasts by inducing Notch signalling
(Mitsiadis et al., 1997;Mustonen et al., 2002). These studies showed that Hes1 expression in the dental precursors is dependent
on mesenchymal signals and that FGF10 induces Hes1
expression in explants of isolated dental epithelium. In these
explants FGF10 also induces the expression of Lunatic fringe, an
enhancer of Notch activity. Interestingly, we have found that
Lunatic fringe is expressed throughout pancreatic development
and that its maximal expression occurs between E12 and E16, a
period corresponding to the expansion of the population of pancreatic
precursors. This period coincides with the maximal expression
of FGF10 by the pancreatic mesenchyme. Moreover,
FGF10 induces Lunatic fringe expression in the E10.5 isolated
dorsal pancreatic epithelium. Thus, in vivo FGF10 could maintain
Notch activity in the pancreatic precursors by inducing Lunatic
fringe.
The present and previous studies indicate that FGF10 is able
to maintain active Notch signalling. However, other signalling
pathways are also probably implicated in Notch control. In this
respect, a recent study has shown that TGFб can induce Notch
activation in explant cultures of pancreatic acinar cells. Upon
treatment with TGFб these cells expressed high levels of Pdx1
and Hes1 and underwent acinar to ductal metaplasia (Miyamoto
et al., 2003). The study of the interactions between the Notch
pathway and other signalling cascades implicated in pancreas
development will be crucial to further unravel the mechanisms
controlling the self-renewal of the pancreatic precursors.
Сайт создан в системе
uCoz  Fig. 1. Effects of FGF10 on the growth and morphogenesis of isolated E10.5 dorsal pancreatic epithelia. (A,B) Isolated pancreatic epithelia grown in vitro for 7 days, without and with 100 ng/ml FGF10. (A) The untreated epithelium fails to grow and remains as a dense agregate of cells with occasional budding. (B) After 7 days of culture, the FGF10-treated epithelium shows an irregular structure formed by buds, cysts and tubules. (C,D) в-catenin/vimentin staining of the pancreatic epithelium after 7 days of culture. (E) Quantitative analysis of the size of the isolated pancreatic epithelia after 3 and 7 days of culture. The bars represent the mean ± SEM of 6 epithelia.
Fig. 1. Effects of FGF10 on the growth and morphogenesis of isolated E10.5 dorsal pancreatic epithelia. (A,B) Isolated pancreatic epithelia grown in vitro for 7 days, without and with 100 ng/ml FGF10. (A) The untreated epithelium fails to grow and remains as a dense agregate of cells with occasional budding. (B) After 7 days of culture, the FGF10-treated epithelium shows an irregular structure formed by buds, cysts and tubules. (C,D) в-catenin/vimentin staining of the pancreatic epithelium after 7 days of culture. (E) Quantitative analysis of the size of the isolated pancreatic epithelia after 3 and 7 days of culture. The bars represent the mean ± SEM of 6 epithelia.