STATS: TRANSCRIPTIONAL CONTROL AND BIOLOGICAL IMPACT David E. Levy & J. E. Darnell
Nature Reviews Molecular Cell
Biology 2002 Vol 3 No 9 Р 651-662
Nature Reviews Molecular Cell Biology 2002 Vol 3 No 9 Р 651-662

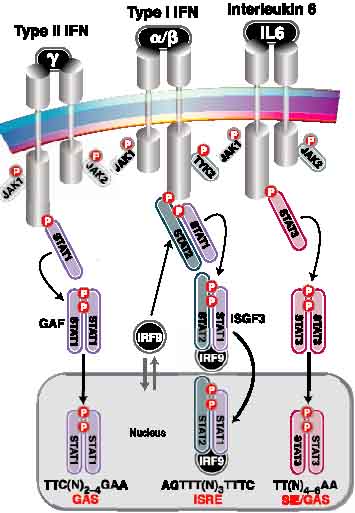
(Рис.1.) | Canonical JAK–STAT pathway.

(Рис.2.) | Janus kinase domain structure.

(Рис.3.) | STAT domain structure and protein binding sites.

(Рис.4.) | The negative regulators of STAT proteins.
(Табл.1) | Role of Janus kinases as revealed by gene-targeting in mice
(Табл.2) |Role of SOCS proteins as shown by mouse genetics
(Табл.3) Role of STAT proteins as revealed by gene-targeting in mice
(Табл.4) Tissue-specific roles of Stat3 as revealed by conditional gene targeting in mice
Boxes

(Box 1.) | Variations in mechanisms of STAT activation
Box 2 | Transcriptional stimulation without phosphorylation
The first indication that signal transducers and activators of transcription (STAT) proteins might have a role in gene expression as unphosphorylated molecules came from experiments using genetically selected human cancer cells in culture that lacked STAT1 (U3A cells). Such cells did not undergo apoptosis upon challenge until STAT1 expression was restored. The U3A cells lacked a full component of , which are present in the U3A cells that express STAT1. However, the apoptotic response did not require wild-type STAT1; a STAT1 Y701F mutant (in which Y is tyrosine and F is phenylalanine), which cannot be phosphorylated on tyrosine, still conferred the ability to respond to apoptotic signals. Subsequently, several other proteins involved in apoptosis were also found to be restored to U3A cells by the STAT1 Y701F mutant.
More recently, gene-array experiments using U3A cells (lacking STAT1) and cells with restored STAT1 were compared without IFN-γ stimulation. A set of genes was found in which the genes were not dependent on IFN and whose mRNAs were present when STAT1 was added back to the U3A cells. One of these genes, termed low molecular weight polypeptide 2 (LMP-2), was studied in detail. STAT1 was constitutively bound to the promoter of this gene in cells not stimulated by IFN and in which no tyrosine phosphorylation of STAT1 could be found. The STAT1 was associated with the promoter, presumably because of its association with interferon regulatory factor-1 (IRF1 — a known binding partner of STAT1) that was also present at the LMP-2 promoter, and can bind DNA on its own. So, it seems clear that unphosphorylated STAT proteins can have a role in transcription, even though it is evident that the main transcriptional stimulation by STATs follows tyrosine phosphorylation, dimerization and nuclear accumulation.
Links
DATABASES
androgen receptor | caveolin-1 | CBP | C/EBP | CIS | c-Jun | EGF | EGF receptor | IFN-α receptor | Ifn-γ | IFN-γ | IFN-γ receptor | IGF-1 | Il-4 | IL-6 | IL-12 | importin-α5 | IRF1 | IRF9 | JAK | Jak1 | JAK1 | leptin | LMP-2 | MCM3 | MCM5 | Nmi | Nup98 | p53 | PIAS1 | PIAS3 | Ptp1b | PTP1B | Socs1 | Stat1 | STAT1 | STAT3 | STAT4 | STAT5A | STAT5B | | TYK2